In Canada, we face critical barriers to truly person-centred HCV care: one important barrier is that people cannot start treatment in the same appointment when their diagnosis is made. This impedes our ability to bring HCV care to priority populations as we work towards eliminating viral hepatitis as a public health threat by 2030.
But it doesn't need to be this way.
Ideally, a patient could get tested for HCV, receive their diagnosis, and start treatment ALL on the same day. This happens in other countries — so why not in Canada? What policies and regulations need to change, and to what technology do we need access in order to be a world leader on this front?
But it doesn't need to be this way.
Ideally, a patient could get tested for HCV, receive their diagnosis, and start treatment ALL on the same day. This happens in other countries — so why not in Canada? What policies and regulations need to change, and to what technology do we need access in order to be a world leader on this front?
Highlights from the Same-Day Starts Discussion. English and French captions provided.
In November 2020, Action Hepatitis Canada and CanHepC co-hosted a virtual panel discussion to bring together clinical and community perspectives as we determine the path forward for Canada.
As hoped, that event has sparked conversation across the country between healthcare providers, community organizations, and policymakers.
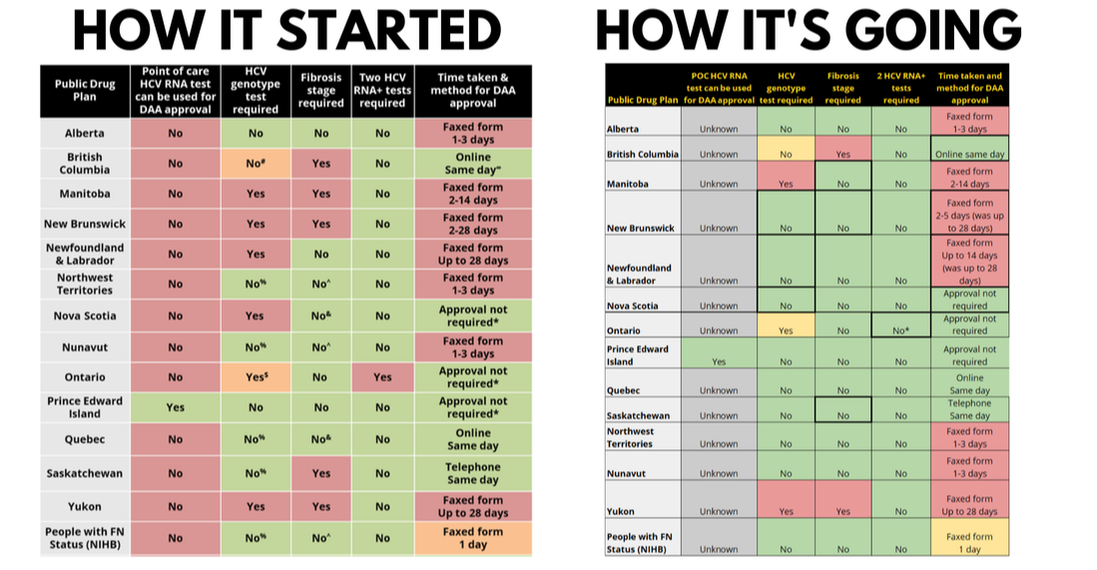
HCV Treatment (DAA) Reimbursement Policies Across Canada
(2021 and 2023 comparison)
A few notes:
- These charts break down the DAA reimbursement policies/potential barriers in each province, territory, and other healthcare provision jurisdiction within Canada.
- We have identified 1 testing technology barrier (RNA Point of Care tests are not yet approved for use here by Health Canada) along with 4 possible policy barriers.
- In order for a patient to be tested, receive a diagnosis, and start treatment in one visit, all 5 columns across would need to be green. Red boxes indicate a policy that causes a delay.
- The first chart appeared in the 2021 Progress Report, the second in the 2023 Progress Report. POlicy barriers continue to be removed, however, the lack of RNA point-of-care testing options remains a significant obstacle.
- Green = good; there's no barrier to same day HCV diagnosis and treatment starts with this policy
- Orange = OK; functionally this policy doesn't create a barrier, but there is a possibility that the policy could become a barrier in the future
- Red = this policy is a barrier to same day HCV diagnosis and treatment starts
Our poster on this topic, Policies For Reimbursement Of Direct-Acting Antiviral Treatment For Hepatitis C Virus Infection In Canada: ‘A Patchwork Of Obstruction’ was displayed at the Canadian Liver Meeting, May 2-5, 2021. The PDF can also be viewed here.
HCV Testing Technology Availability in Canada
One critical obstacle to same-day HCV treatment starts is that there is no Health Canada-approved point-of-care HCV confirmatory molecular test, such as the Cepheid Xpert® HCV Viral Load or Xpert® HCV VL Fingerstick assays.
While there have been several settings throughout Canada where these Xpert® HCV cartridges have been imported and used under Research Use Only (RUO) or Health Canada Special Access permits, unfortunately, these access routes do not adequately meet the need. The publicly-funded drug plans in Canada (which cover up to 80% of people living with HCV in this country) require recent HCV RNA test results to be provided with treatment reimbursement requests. Only results from Health Canada-approved diagnostic tests or Public Health Lab-Accredited tests are accepted for this purpose.
Our current centralized HCV testing process particularly impedes our ability to bring HCV care to priority populations, many of whom face barriers to accessing mainstream healthcare.
Cepheid received approval in January 2021 for the Xpert® Xpress SARS-CoV-2/Flu/RSV from Health Canada. The GeneXpert System, now being further distributed across Canada to assist in addressing COVID-19, holds great opportunity for point-of-care diagnostics beyond this pandemic. In May 2021, AHC wrote to Cepheid asking them to prioritize HCV test cartridges for the GeneXpert System for submission to Health Canada.
While there have been several settings throughout Canada where these Xpert® HCV cartridges have been imported and used under Research Use Only (RUO) or Health Canada Special Access permits, unfortunately, these access routes do not adequately meet the need. The publicly-funded drug plans in Canada (which cover up to 80% of people living with HCV in this country) require recent HCV RNA test results to be provided with treatment reimbursement requests. Only results from Health Canada-approved diagnostic tests or Public Health Lab-Accredited tests are accepted for this purpose.
Our current centralized HCV testing process particularly impedes our ability to bring HCV care to priority populations, many of whom face barriers to accessing mainstream healthcare.
Cepheid received approval in January 2021 for the Xpert® Xpress SARS-CoV-2/Flu/RSV from Health Canada. The GeneXpert System, now being further distributed across Canada to assist in addressing COVID-19, holds great opportunity for point-of-care diagnostics beyond this pandemic. In May 2021, AHC wrote to Cepheid asking them to prioritize HCV test cartridges for the GeneXpert System for submission to Health Canada.
New! INHSU released this report on
Barriers and Solutions to Point-of-Care Testing in January 2024.
Barriers and Solutions to Point-of-Care Testing in January 2024.